Blog
Step in Time
17 December 2020
 MIT
MITMeasuring time is about counting steps. Whether it’s the drip-drip of a water clock, the tic-toc of a mechanical clock, or the oscillating crystal of a quartz watch. Any accurate timepiece is built around counting the steps of something regular and periodic. Nothing is perfectly regular, so no clock keeps perfect time, but our timepieces are getting very, very accurate.
Time has traditionally be measured in terms of astronomy, such as the rising and setting of the Sun, or the motion of the stars and Moon. There are have been several popular methods, but by the 1800s time was measured by what is known as the mean solar day. A day isn’t always exactly 24 hours long. As the Earth moves along its elliptical orbit, its speed around the Sun changes slightly, making the day slightly longer or shorter depending on the season. But by taking the average (specifically the mean) of days over a year, astronomers could define a common standard.
As our mechanical clocks got more accurate, it became clear that the mean solar day was problematic. The rotation of the Earth isn’t constant but changes due to tectonic shifts, and because of its gravitational dance the with Moon, the Earth rotates more slowly over time. So in 1956 time was defined in terms of the orbit of the Earth rather than its rotation.
 US Naval Observatory
US Naval ObservatoryIn 1967, we moved away from astronomy altogether when the length of a second was redefined in terms of an atomic clock. The measured oscillation is based not on an atom, but the light emitted by an atom. Atoms create light when an electron moves from a higher energy state to a lower one. Since energy levels in an atom are quantized, light is emitted at a precise frequency. In 1967 length of a second was set by defining a specific emission from caesium-133 to be exactly 9,192,631,770 Hz. This is the standard we still use today.
Although the modern standard is officially exact, it isn’t actually exact. Two atomic clocks of the same design keep slightly different times. By statistically comparing atomic clocks, we know they are accurate to about one second in thirty million years. That’s probably accurate enough for everyday use, but it isn’t accurate enough for some scientific purposes. If we had more precise clocks, we could use them to study everything from geology to dark energy. So there is an ongoing quest to develop a new, more accurate standard.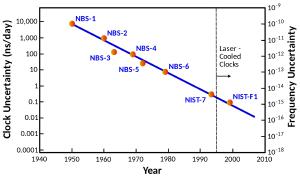 Wikipedia
Wikipedia
Most of the approaches look toward purely optical methods, but new work in Nature uses atoms in quantum entanglement.1 One of the reasons modern atomic clocks aren’t perfect is that the atoms recoil when light is emitted, which shifts the frequency of emitted light slightly. If the atom could be kept perfectly stationary when it emits light, then the frequency of the light would be exact. But quantum mechanics keeps the position of an atom a bit fuzzy, meaning that the frequency of emitted light is also a bit fuzzy. This effect is known as the standard quantum limit.
To address this problem, the team uses an effect known as quantum entanglement. By using lasers to squeeze atoms together, they can force the atoms to interact on a quantum level such that a measurement on one atom also measures all of them. Thus, the states of these atoms are entangled. When another laser is used to trigger an atom into emitting light, a cascade occurs that syncs the atoms together. The light emitted is therefore much more precise than the standard quantum limit.
Statistical analysis of this new clock shows that it can function within an accuracy of 100 milliseconds over the age of the universe. The clock is so precise that it could test whether universal physical constants change over time.
Our standard for timekeeping won’t change soon, but it is clear we can do better. At some point in the future, we will adopt a more accurate method. When we do, a clock based upon quantum entanglement could be the solution. If that’s the case, our official clocks will use quantum weirdness to overcome quantum fuzziness.
Edwin Pedrozo-Peñafiel, et al. “Entanglement on an optical atomic-clock transition.” Nature 588 (2020): 414 ↩︎